Filed pursuant to Rule 424(b)(3)
SEC File No. 333-268297
PROSPECTUS SUPPLEMENT NO. 2
(to Prospectus dated November 25, 2022)
MediWound Ltd.
28,153,058 ORDINARY SHARES
This prospectus supplement updates, amends and supplements the prospectus contained in our Registration Statement on Form F-1, effective as of
November 25, 2022 (as supplemented or amended from time to time, the “Prospectus”) (Registration No. 333-268297). Capitalized terms used in this prospectus supplement and not otherwise defined herein have the meanings specified in the Prospectus.
This prospectus supplement is being filed to update, amend and supplement the information included in the Prospectus with information set forth
below.
This prospectus supplement is not complete without the Prospectus. This prospectus supplement should be read in conjunction with the Prospectus,
which is to be delivered with this prospectus supplement, and is qualified by reference thereto, except to the extent that the information in this prospectus supplement updates or supersedes the information contained in the Prospectus. Please
keep this prospectus supplement with your Prospectus for future reference.
Our ordinary shares are listed on the Nasdaq Stock Market LLC under the trading symbols “MDWD.” On January 6, 2023, the closing price for our
ordinary shares on the Nasdaq Stock Market LLC was $12.64 per ordinary share.
Investing in our securities involves a high degree of risk. See “Risk Factors” beginning on page 8 of the Prospectus and
other risk factors contained in the documents incorporated by reference therein for a discussion of information that should be considered in connection with an investment in our securities.
Neither the Securities and Exchange Commission, the Israeli Securities Authority nor any state securities commission has approved
or disapproved of these securities or determined if the Prospectus or this prospectus supplement is truthful or complete. Any representation to the contrary is a criminal offense.
The date of this prospectus supplement is January 9, 2023.
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
REPORT OF FOREIGN PRIVATE ISSUER
Pursuant to Rule 13a-16 or 15d-16 of the
Securities Exchange Act of 1934
For the month of January 2023
Commission File Number: 001-36349
MediWound Ltd.
(Translation of registrant’s name into English)
42 Hayarkon Street
Yavne, 8122745 Israel
(Address of principal executive offices)
Indicate by check mark whether the registrant files or will file annual reports under cover Form 20-F or Form 40-F.
Form 20-F ☒ Form 40-F ☐
|
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b) (1): __
|
| |
|
|
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b) (7): __
|
CONTENTS
On January 9, 2023, MediWound Ltd. (the “Company”) made a presentation at the J.P. Morgan 41st Annual Healthcare Conference,
highlighting its clinical products, and providing certain updates regarding the results of clinical trials, as well as certain estimates and projections as to expected future financial results. Materials used in conjunction with the presentation
are available on the Company’s website at www.mediwound.com and are furnished as Exhibit 99.1 to this Report of Foreign Private Issuer on Form 6-K (this “Form 6-K”). The contents of the foregoing
website are not a part of this Form 6-K.
The information contained in the presentation was provided as of January 9, 2023, and the Company does not undertake any obligation to update the presentation in the future or to update
forward-looking statements to reflect subsequent actual results. The furnishing of the materials related to the presentation is not an admission as to the materiality of any information contained in those materials.
The contents of this Form 6-K (including the information contained in Exhibit 99.1) are hereby incorporated by reference into the Company’s Registration Statements on Form S-8,
filed with the SEC on April 28, 2014, March 24, 2016, March 19, 2018, March 25, 2019, February 25, 2020, May 15, 2021 and August 9, 2022 (Registration Nos. 333-195517, 333-210375, 333-223767, 333-195517, 333-210375, 333-230487, 333-236635,
333-255784, and 333-266697, respectively), and Form F-3, filed with the SEC on May 25, 2022 (Registration No. 333-265203).
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
Date: January 9, 2023
|
MEDIWOUND LTD.
By: /s/ Boaz Gur-Lavie
Name: Boaz Gur-Lavie
Title: Chief Financial Office
|
EXHIBIT INDEX
The following exhibit is furnished as part of this Form 6-K:
4
January 2023 I Nasdaq: MDWD Non-Surgical Biological Solutions for
Tissue Repair & Regeneration
2 Cautionary Note Regarding Forward-Looking Statements This
presentation contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act and other securities laws, including but not limited to the statements related to the commercial potential of
our products and product candidates, the anticipated development progress of our products and product candidates, and our expected cash runaway. In some cases, you can identify forward-looking statements by terminology such as
“believe,” “may,” “estimate,” “continue,” “anticipate,” “intend,” “should,” “plan,” “expect,” “predict,” “potential,” or the negative of these terms or other similar expressions. Forward-looking statements are not historical
facts, and are based upon management’s current expectations, beliefs and projections, many of which, by their nature, are inherently uncertain. Such expectations, beliefs and projections are expressed in good faith. However,
there can be no assurance that management’s expectations, beliefs and projections will be achieved and actual results may differ materially from what is expressed in or indicated by the forward-looking statements. Important
factors that could cause such differences include, but are not limited to the uncertain, lengthy and expensive nature of the product development process; market acceptance of our products and product candidates; the timing and
conduct of our studies of our product candidates; our ability to obtain marketing approval of our products and product candidates in the U.S. or other markets; our expectations regarding future growth, including our ability to
develop new products; risks related to our contracts with BARDA; our ability to maintain adequate protection of our intellectual property; competition risks; and the need for additional financing. These and other significant
factors are discussed in greater detail in MediWound’s annual report on Form 20-F for the year ended December 31, 2021, filed with the Securities and Exchange Commission (“SEC”) on March 17, 2022, and other filings with the SEC
from time-to-time. These forward-looking statements reflect MediWound’s current views as of the date hereof and MediWound undertakes, and specifically disclaims, any obligation to update any of these forward-looking statements
to reflect a change in their respective views or events or circumstances that occur after the date of this release except as required by law Certain studies and data presented herein have been conducted for us by other entities
as indicated where relevant. Intellectual property, including patents, copyrights or trade secret displayed in this presentation, whether registered or unregistered, are the intellectual property rights of MediWound. MediWound's
name and logo and other MediWound product names, slogans and logos referenced in this presentation are trademarks of MediWound Ltd. and/or its subsidiaries, registered in the U.S.A., EU member states and Israel. NexoBrid
development has been supported in part with federal funding from U.S. Biomedical Advanced Research and Development Authority (BARDA), Administration for Strategic Preparedness and Response (ASPR), within the U.S. Department of
Health and Human Services (HHS), under ongoing USG Contract numbers HHSO100201500035C and HHSO100201800023C. Contract number HHSO100201500035C provides funding and technical support for the pivotal U.S. Phase 3 clinical study
(DETECT) and the marketing approval registration process for NexoBrid as well as its procurement and availability under the expanded access treatment protocol (NEXT) in the U.S. Additional projects for evaluation of NexoBrid
funded under the BARDA contract include randomized, controlled pivotal clinical trial for use in pediatric population, establishment of a pre-emergency use data package and development of the health economic model to evaluate
the cost savings impact to enable market adoption in the United States. We maintain our books and records in U.S. dollars and report under IFRS.
3 Company Highlights Diversified portfolio Global strategic
collaborations cGMP certified sterile manufacturing facility Solid balance sheet & strong investor base NexoBrid® - 2022 revenues: $26-27M EscharEx® - $2B* opportunity Validated enzymatic technology
platform FDA/EMA/PMDA approvals 14 successful clinical trials 120+peer reviewed publications Provides capacity to scale revenue growth BARDA, Vericel, DoD (US), Kaken (JP), BSV (IN) $42M cash Runway through 2025 *TAM -
targeted addressable market; Source: Oliver Wyman market research
4 Leadership Team Nachum (Homi) Shamir Chairman of the
Board Ofer Gonen Chief Executive Officer Dr. Ety Klinger Chief R&D Officer Tzvi Palash Chief Operating Officer Boaz Gur-Lavie Chief Financial Officer Dr. Robert J. Snyder Chief Medical Officer Prof. Lior
Rosenberg Founder, Medical Director
5 Proprietary Enzymatic Technology Platform Pineapple stem
harvest Protein extraction Purification, enrichment, stabilization Complex mixture of proteolytic enzymes Images modified from Labster theory and bioinfo Healthy skin Damaged skin Selective enzymes target only non-viable
tissue Viable tissues preserved; healing begins Clinically and commercially validated protein-based therapies
6 Multibillion Dollar Portfolio Indication: Eschar removal of deep
partial and full thickness burns Classification: Orphan biological drug Target users: Hospitalized patients Substantial U.S. government support Development status: FDA/EU/JP approved NexoBrid® Disruptive therapy for burn
care Indication: Debridement of chronic / hard-to-heal wounds Classification: Biological drug Optimized for outpatient setting Development status: Phase III ready EscharEx® Next-gen enzymatic therapy for wound
care** Indication: Treatment of non-melanoma skin cancers Classification: Biological drug Optimized for outpatient setting Development status: Phase I/II MW005 Biotherapy for non-melanoma skin
cancers** Pipeline Commercial *TAM - targeted addressable market; Source: Oliver Wyman market research **Investigational drug TAM* (U.S.): >$2B >$200M TAM* (U.S.): >$1B TAM* (U.S.): Pipeline
7 Upcoming Milestones 2023 Phase I/II results MW005 Phase I/II
data 2024 NexoBrid® EU Pediatric label extension approval FDA approval FDA Pediatric label extension submission Marketing approval in JP/ IN US commercial launch Meetings with FDA EscharEx® CHMP Scientific
advice Phase III initiation Phase II results JP commercial launch
Financial Highlights 8 * Cash, cash equivalents and short-term
bank deposits; cash amount takes into account the receipt of $7.5M milestone from Vericel upon BLA approval $42M in cash* as of December 31, 2022 Cash runway - through 2025 Strong investor base BALANCE SHEET 2022 revenues
of ~$26-27M NexoBrid is profitable 2023 Product revenues >75% growth 2023 Product gross margin >50%; scale-up drives further increase REVENUES Global expansion via strategic collaborations (Vericel, Kaken, BSV,
GAG) Up to $211M support by BARDA EU direct sales force (CAGR >20%) COMMERCIALIZATION MDWD ANALYSTS: Josh Jennings, MD, Cowen Jacob Hughes, Wells Fargo Francois Brisebois, Oppenheimer Swayampakula Ramakanth, PhD,
HCW David Bouchey, Aegis Jason McCarthy, Ph.D, Maxim
Early, effective and selective non-surgical eschar removal for
severe burns Globally approved: FDA, EU, JP, IN; 11,000 patients Validated & commercialized
10 Clear Unmet Need for Early, Effective and Selective Non-Surgical
Eschar Removal in Severe Burns Eschar Removal is the 1st Critical Step in Burn Care Loss of healthy tissue & blood Current Practice* is Traumatic & non-selective Prevents local infection and
sepsis Eschar Requires surgical team, operating room Challenging in delicate areas Avoids further deterioration and scarring Enables initiation of wound healing Allows visual assessment of wound bed *current
non-surgical eschar removal has limited efficacy, and requires multiple dressing changes
11 Indicated for eschar removal of deep-partial &
full-thickness thermal burns A sterile mixture of proteolytic enzymes Effectively removes eschar within 4 hours without harming viable tissue or blood loss Allows for early visual assessment of the
wound Before After Disruptive Bioactive Therapy for Burn Care Significantly reduces need for surgery & improves patient outcomes Easy-to-use, topical application at patient’s bedside
12 NexoBrid® - Phase III Studies Demonstrate Superiority Incidence
of complete eschar removal P<0.0001 Time to complete eschar removal P<0.0001 NexoBrid N=75 SOC N=75 1.0 0.5 0.0 0 10 20 30 40 Incidence of surgical eschar removal P <0.0001 Blood
loss P<0.0001 NexoBrid® SOC SOC® NexoBrid® SOC NexoBrid® Gel Vehicle [N=175] [N=175] [N=175] [N=175] Consistent with EU Phase III study & pediatrics Phase III study No safety issues after 24 month
follow-up Non-inferiority in time to complete wound closure & scarring NexoBrid® SOC 815ml 14ml
Next-Generation Enzymatic Debridement for Wound Care Superior to
SOC - Sets a new bar for efficacy De-risked: based on a validated technology Targets $2b market opportunity
Modalities by Efficacy and Convenience Modalities by Wound Type
(U.S.)* Efficacy Trained Specialist Untrained HCP/ Nurses Ultrasonic Versajet Biological Sharp Enzymatic Autolytic 29% 29% Legend 14 Approaches in Chronic Wound Debridement are abundant but sub-optimal *Source:
OW Primary Research (6/2022) | VLU – Venus Leg Ulcers | DFU – Diabetic Foot Ulcer
15 Indicated for debridement of chronic and hard-to-heal
wounds Investigational drug containing a sterile mixture of proteolytic enzymes Debrides chronic wounds in 4-6 daily applications Next-Generation Enzymatic Debridement - Wound Bed Preparation within a Week Inline with
current treatment workflows and reimbursement landscape Easy to use, daily topical application for outpatient setting Extended IP protection *Investigational Drug, not approved in any jurisdiction *Investigational Drug, not
approved in any jurisdiction VLU Before After Before After DFU ®
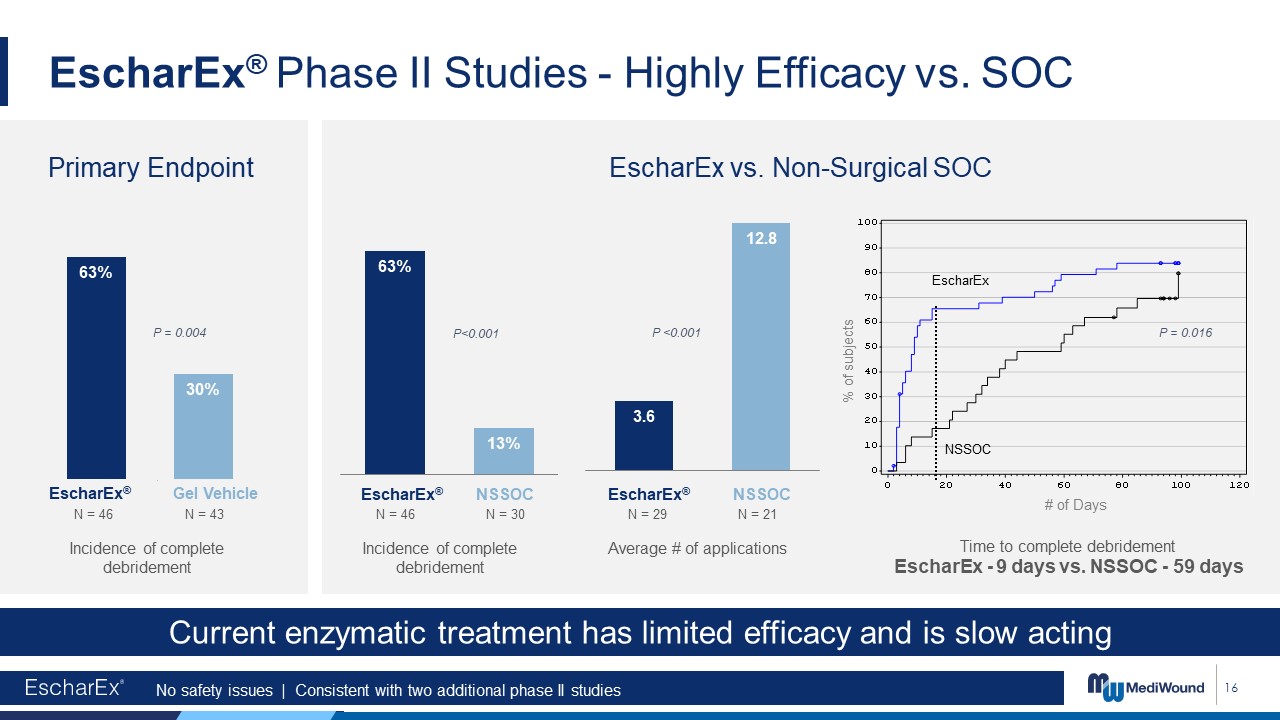
16 Average # of applications Time to complete debridement EscharEx
- 9 days vs. NSSOC - 59 days P<0.001 P = 0.016 # of Days EscharEx NSSOC % of subjects Incidence of complete debridement EscharEx vs. Non-Surgical SOC Incidence of complete debridement P <0.001 No safety issues |
Consistent with two additional phase II studies Current enzymatic treatment has limited efficacy and is slow acting EscharEx® Phase II Studies - Highly Efficacy vs. SOC Primary Endpoint P = 0.004 EscharEx® Gel
Vehicle EscharEx® NSSOC EscharEx® NSSOC N = 46 N = 43 N = 46 N = 30 N = 29 N = 21
17 *Source: OW Primary Research (6/2022) 29% 55% Market
potential growth EscharEx® anticipated to draw share from all other debridement modalities 2.1M patients VLU: 1.0M | DFU: 1.1M 400K patients 1.3M patients VLU: 560K DFU: 770K 2022 Epidemiology Estimate TAM - $2B 30%
expected market share 70% eligible debridement EscharEx® U.S. Market Opportunity Cost of treatment: 1,500-1,800$*
Novel biotherapy for Non-Melanoma Skin Cancer MW005 Effective and
safe topical application BCC is the most diagnosed skin cancer in the US
19 Novel Biotherapy for Non-Melanoma Skin
Cancer MW005 Before After The Market 4.3M annual cases of Basal Cell Carcinomas diagnosed in the US Surgery is the SOC; topical products have high AEs & recurrence rates The Product Investigational drug containing a
sterile mixture of proteolytic enzymes Easy to use, high potency, 5-7 topical applications US Phase I/II study, demonstrated efficacy, safety and tolerability MW005
$26-27M revenues mainly from non-products NexoBrid® FDA
approved $42M in cash* Robust EscharEx® Phase II results 20 Why MediWound? 2022 2023 2025 2026 EscharEx® Phase III initiation Scale-up manufacturing facility NexoBrid® Product revenue growth >75% $40-50M
Revenues from products Additional revenues (BARDA, DoD) Gross Margin >60% EscharEx® approval Cashflow positive >$100M Revenues with contribution from EscharEx® * Cash amount takes into account the receipt of $7.5M
milestone from Vericel upon BLA approval